|
|
|
Select a Color
|
|
Creation date: Feb 22, 2024 11:26pm Last modified date: Feb 22, 2024 11:26pm Last visit date: Jul 14, 2024 10:36am
1 / 20 posts
Feb 22, 2024 ( 1 post ) 2/22/2024
11:26pm
Wang Meihong (chenluseo)
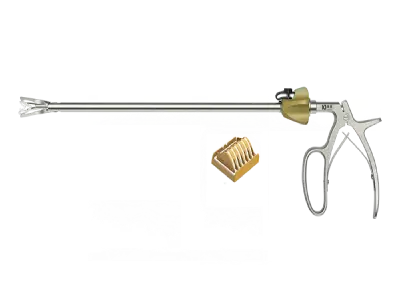
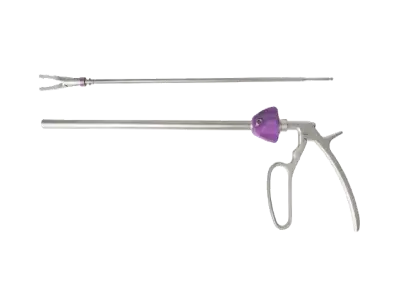
Polymer clip appliers can be used in all kinds of laparoscopic surgery, including minimally invasive surgery, minimally invasive gynecological surgery, thoracic surgery, urology, endoscopic surgery. In the ever-expanding market of China, several suppliers stand out for their commitment to quality, innovation, and customer satisfaction. In this article, we will explore the top five polymer clip applier suppliers in China.
What Is a Polymer Clip?A polymer clip refers to a type of clip or fastener made from polymer materials, which are high-quality, durable, and often biocompatible synthetic substances. Polymer clips find applications in various industries, including medical, packaging, and manufacturing, due to their versatility and advantageous properties.
In the medical field, polymer clips are commonly used in surgeries and medical procedures. Specifically, they are utilized in minimally invasive surgical techniques, where surgeons require tools that are lightweight, easy to handle, and compatible with medical imaging technologies. These clips are designed for tasks such as ligation or closure of blood vessels, tissues, or other structures during surgical procedures. Are Polymer Clips Absorbable?Polymer clips can be both absorbable and non-absorbable, depending on the specific type of polymer used in their construction. Absorbable polymer clips are designed to degrade or be absorbed by the body over time, eliminating the need for removal after a certain healing period. These clips are often used in medical applications where a temporary closure or ligation is required.
Common materials for absorbable polymer clips include polymers such as polylactic acid (PLA) or polyglycolic acid (PGA). These polymers are known for their biocompatibility and ability to break down into non-toxic byproducts that the body can naturally absorb. On the other hand, non-absorbable polymer clips are made from materials that do not break down within the body and are intended for long-term use. They are often used in situations where a permanent closure or ligation is required. Common non-absorbable polymers used for clips include polyethylene and polypropylene. In medical procedures, absorbable polymer clips are typically used in applications where the closure or ligation is temporary, and the clips will naturally degrade as the tissues heal. Non-absorbable polymer clips are used when a more permanent solution is needed, such as in certain types of surgeries where long-term support or closure is required. It's essential to note that the choice between absorbable and non-absorbable polymer clips depends on the specific requirements of the medical procedure and the expected healing timeline. Surgeons will consider factors such as the type of surgery, patient condition, and the duration of clip retention when selecting the appropriate type of polymer clip for a particular application. China Polymer Clip Applier Suppliers1.Hangzhou Kangji Medical Instrument Co. Ltd Kangji Medical, founded in 2004 and headquartered in Hangzhou, Zhejiang Province, China, is a prominent medical device group. In June 2020, we achieved a milestone by being listed on the mainboard of the Stock Exchange of Hong Kong (Stock Code: 9997.HK). Specializing in the design, development, manufacturing, and sale of minimally invasive surgery instruments and accessories (MISIA), Kangji Medical is driven by a mission to "provide physicians with high-quality products and services, dedicated to improving people's health."
Our focus extends to delivering comprehensive surgical solutions tailored for four major specialties: obstetrics and gynecology, general surgery, urology, and thoracic surgery. Kangji Medical strives to create a one-stop platform for physicians and hospitals, offering a diverse product portfolio. We are dedicated to establishing a globally recognized presence in minimally invasive surgery instruments and accessories. To support the development of MISIA products that align with clinical needs, Kangji Medical has established research platforms, including a Provincial Academician Research Workstation, Provincial Enterprise Research Institute, and an Enterprise High and New Technology Research Centre. This strategic approach ensures that our product portfolio is both comprehensive and solution-oriented, ultimately enhancing surgical efficiency and improving clinical outcomes for patients. In recognition of our commitment to quality control, Kangji Medical has earned international accreditations, including EN ISO 13485:2016, ISO 9001:2015, ISO 45001:2018, ISO 14001:2015, and CE(93/42/EEC). These certifications underscore our dedication to maintaining high standards and ensuring the safety and efficacy of our minimally invasive surgery instruments and accessories. 2.Hangzhou Sunstone Technology Co., Ltd Established in 2005, Hangzhou Sunstone Technology Co., Ltd. is a manufacturing enterprise with a steadfast commitment to the research and development, as well as the sales, of innovative medical devices. Situated in Hangzhou, China, the enterprise park spans over 7,000 square meters, with assets exceeding 30 million US dollars. Within this expansive facility, the production and office area covers 20,000 square meters, inclusive of an ISO 8 class cleanroom surpassing 1,000 square meters and an ISO 7 class cleanroom exceeding 500 square meters.
Sunstone Technology proudly holds the distinction of being the first national high-tech enterprise in Zhejiang Province, China, dedicated to the research and development of absorbable medical consumables. The company has earned the qualification of a high-tech enterprise research and development center from the Zhejiang Province Science and Technology Department of Innovation Medical Devices. The portfolio boasts several unique medical devices, each of which has been submitted for both Chinese and international patents. These groundbreaking projects are at various stages of research and development, with some progressing into the registration and approval phase in China, while others are undergoing EU CE certification. At Hangzhou Sunstone Technology Co., Ltd., the dedication to advancing medical technology is evident in the continuous pursuit of innovation and excellence. 3.Hangzhou Valued MedTech Co., Ltd Founded in 2012, Hangzhou Valued MedTech Co., Ltd. has established itself as a leading medical supplier company in China, specializing in the manufacturing of laparoscopic instruments. The company earned the prestigious title of "National High-tech Enterprise" from China's Ministry of Science and Technology in 2019.
With an expansive 5,000 square meters of space, the state-of-the-art factory includes a 500-square-meter dust-free workshop. The guiding principle of "striving for excellence" inspires all workers to produce high-quality products, while dedicated employees are committed to providing unparalleled customer service. Over time, the company has diversified its product range, and each item in the portfolio is certified by ISO, CE, and FDA standards. The product lineup includes disposable laparoscopic trocars, trocar kits, endo bags, endoloops, ligation clips, laparoscopic pencil hooks, laparoscopic graspers/tissue forceps, bipolar forceps, camera sleeves, and more. Hangzhou Valued MedTech Co., Ltd. remains dedicated to advancing medical technology and enhancing patient care through its comprehensive range of quality laparoscopic instruments. 4.Hangzhou Boer Medical Instrument Co., Ltd. Boer, a dedicated professional manufacturer and supplier, is actively involved in the research and development, design, production, and sales of a diverse range of disposable minimally invasive surgical (MIS) instruments. The company holds the esteemed ISO13485 and CE certificates, signifying its commitment to quality, and has successfully navigated the latest MDR audit.
The extensive product portfolio includes disposable laparoscopic trocars, disposable laparoscopic forceps/scissors, disposable Veress needles, disposable suction irrigation tubes, disposable specimen retrieval bags, and various other disposable surgical instruments.
Behind the innovation and quality of Boer's products are Research and Development engineers from the leading medical instrument company in China. The skilled craftsmanship of the workers contributes to the precision and reliability of the instruments. With professional exporting experience, Boer ensures seamless transactions for its clients, backed by heartfelt pre-sales and after-sales service.
5.Jiangsu BANA Medical Technology Co., Ltd. Established in 1993, Jiangsu BANA Medical Technology Co., Ltd. ("JSBANA") has focused on the production of laparoscopic instruments, endoscopic products, orthopedic implant trauma products, spinal systems, and implanted joints, among others.
As a professional manufacturer and integrated solution provider, JSBANA prioritizes Research and Development, manufacturing, sales, and service. The company takes pride in offering both OEM and ODM services to meet the diverse needs of its clients. JSBANA operates from a modern standard workshop covering over 46,000 sqm and a Class-100,000 cleanroom spanning over 1,000 sqm. With a dedicated team of over 200 employees, the company ensures quality and precision in every product manufactured. Certified with CE, ISO13485, Chinese FDA registration, and Free Sales Certificates, JSBANA holds the distinction of being recognized as a High-Tech Enterprise and High-Tech Products in Jiangsu Province. Several OEM products from JSBANA have received FDA registration, and its reach extends globally with exports to over 60 countries and districts. At JSBANA, the commitment to quality, innovation, and global impact has remained unwavering since its establishment in 1993. |